Abstract
Background: Our institution routinely performs autologous back-up haematopoietic progenitor cell (HPC) harvest prior to unrelated donor transplantation, particularly in the post-pandemic era, or in other circumstances if there is risk of graft failure. This practice is influenced by our relative geographical isolation and significant challenges in obtaining emergency allogeneic products. As the treatment landscape for paediatric acute lymphoblastic leukaemia (ALL) has expanded to incorporate immunotherapy, many patients proceeding to transplant receive the bispecific T-cell engager blinatumomab. Little is known about HPC mobilisation outcomes in patients following blinatumomab therapy. Here we report our experience with HPC mobilisation for autologous back-up harvest in paediatric patients with ALL treated with blinatumomab.
Methods: We reviewed HPC mobilisation attempts in patients with ALL at The Children's Hospital at Westmead, Sydney, Australia from 1 January 2012 to 30 June 2022. Patients were analysed in two groups: those mobilised following blinatumomab and those mobilised following chemotherapy or from steady state. Patients were mobilised according to our institutional practice. Following chemotherapy or blinatumomab, granulocyte-colony stimulating factor (G-CSF) was given subcutaneously at a dose of 5 µg/kg/day after chemotherapy, or 10 µg/kg/day after blinatumomab or from steady state haematopoiesis. Records were assessed retrospectively for demographics, mobilisation strategy, G-CSF days, apheresis days, final HPC yield in CD34+ cells/kg, transplant characteristics, and outcomes. Successful mobilisation was defined as proceeding to apheresis and successful harvest at the patient level by achieving an HPC yield of at least 2 x 106 CD34+ cells/kg.
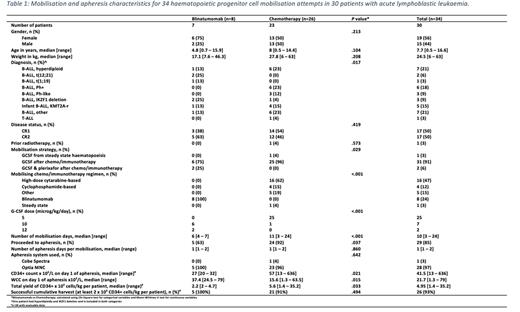
Results: Over the 10.5 year (y) study period, 30 patients with ALL underwent 34 HPC mobilisation attempts for the purpose of autologous back-up HPC harvest prior to allogeneic transplant; 7 (23%) after blinatumomab and 23 (77%) after chemotherapy. Mobilisation and apheresis characteristics of the two groups are summarised in Table 1. There were no differences in age, weight, gender, disease status or prior exposure to radiotherapy between the blinatumomab and chemotherapy groups. Successful mobilisation was less likely in the blinatumomab group with 5/8 (63%) proceeding to apheresis vs 24/26 (92%) in the chemotherapy group, P=.037. In 29 successful mobilisations, the blinatumomab group had a lower median number of days of G-CSF (6 (range 5-7) vs 11 (3-24), P=.006), and greater utilisation of plerixafor (2/5 (40%) vs 0/24 (0%), P=.001). On day 1 of apheresis, the blinatumomab group had a lower median CD34+ count x 106/L (27 (20-32) vs 57 (13-636), P=.021) and a higher median white cell count x 109/L (37.4 (24.5-79) vs 15.6 (1.3-63.5), P=.015). Although the median total HPC yield of CD34+ cells x 106/kg was significantly lower in the blinatumomab group (2.2 (2-4.7) vs 5.6 (1.4-35.2), P=.033), there was no difference in the proportion of patients achieving the collection target of 2 x 106 CD34+ cells/kg (5/5 (100%) vs 21/23 (91%), P=.494). With a median follow up of 1.6y for the blinatumomab group and 7y for the chemotherapy group, there was no difference in 2y relapse-free (83% vs 57%, P=.310) or overall (83% vs 67%, P=.5903) survival between the groups.
Of 28 patients who proceeded to transplant, 3 (11%) required autologous HPC reinfusion for secondary graft failure, 2/3 after 9/10 matched unrelated donor transplants without serotherapy, and all from the chemotherapy group. Neutrophil engraftment for these 3 patients occurred at a median of 13 days (range 11 - 14). One patient died 35 days after salvage reinfusion without achieving platelet engraftment. The 2 surviving patients achieved platelet engraftment at 18 and 29 days post reinfusion and remain alive in continuous complete remission more than 3y following HPC re-infusion.
Conclusions: HPC mobilisation for autologous back-up harvest is feasible for ALL patients following blinatumomab. However, these patients are at risk of failed mobilisation and may benefit from pre-emptive strategies such as the use of plerixafor to optimise mobilisation success. Our relatively high rate of salvage reinfusion of autologous HPCs in ALL patients underlines the utility of universal back-up harvests for patients undergoing unrelated donor transplant.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.